
Liver Cirrhosis Clinical Trial Pipeline Experiences Momentum: DelveInsight Estimates a Diverse Pipeline Comprising 30+ Companies Working in the Domain
The liver cirrhosis treatment market is poised for significant growth, driven by the rising global prevalence of liver diseases, particularly NAFLD, fueled by lifestyle factors like obesity, alcohol consumption, and metabolic syndrome. Technological advancements in antiviral therapies and regenerative medicine are enhancing treatment outcomes, while an aging population further increases demand. These factors collectively highlight a strong growth trajectory for the market.
/EIN News/ -- New York, USA, Jan. 15, 2025 (GLOBE NEWSWIRE) -- Liver Cirrhosis Clinical Trial Pipeline Experiences Momentum: DelveInsight Estimates a Diverse Pipeline Comprising 30+ Companies Working in the Domain
The liver cirrhosis treatment market is poised for significant growth, driven by the rising global prevalence of liver diseases, particularly NAFLD, fueled by lifestyle factors like obesity, alcohol consumption, and metabolic syndrome. Technological advancements in antiviral therapies and regenerative medicine are enhancing treatment outcomes, while an aging population further increases demand. These factors collectively highlight a strong growth trajectory for the market.
DelveInsight’s 'Liver Cirrhosis Pipeline Insight 2025' report provides comprehensive global coverage of pipeline liver cirrhosis therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the liver cirrhosis pipeline domain.
Key Takeaways from the Liver Cirrhosis Pipeline Report
- DelveInsight’s liver cirrhosis pipeline report depicts a robust space with 30+ active players working to develop 30+ pipeline liver cirrhosis drugs.
- Key liver cirrhosis companies such as Versantis AG, Mirum Pharmaceuticals, Sagimet Biosciences, TenNor Therapeutics, Prism Pharma, Vedanta Biosciences, Lipocine, Gwo Xi Stem Cell Applied Technology, Galectin Therapeutics, Resolution Therapeutics, Ipsen, AstraZeneca, and others are evaluating new liver cirrhosis drugs to improve the treatment landscape.
- Promising pipeline liver cirrhosis therapies such as Volixibat, VS-01, TVB-2640, TNP-2092, PRI-724, VE303, LPCN 1148, GXHPC1, Belapectin, RTX001, Elafibranor, Zibotentan, and others are under different phases of liver cirrhosis clinical trials.
- In December 2024, Galectin Therapeutics announced results from its global clinical trial NAVIGATE evaluating belapectin in patients with Metabolic Dysfunction-Associated Steatohepatitis (MASH) cirrhosis and portal hypertension.
- In October 2024, PharmaIN Corporation announced the company will present interim results from its ongoing Phase I clinical trial of PHIN-214, the company's lead candidate, for the prevention and treatment of decompensated cirrhosis.
- In June 2024, Resolution Therapeutics Limited announced key data presentations of RTX001 with the University of Edinburgh at the EASL Congress 2024, held in Milan, Italy which demonstrate the significant potential of macrophage cell therapy as a treatment for advanced liver cirrhosis.
- In June 2024, Lipocine announced that Phase II results on LPCN 1148 in cirrhosis were featured in a late breaking oral presentation at the European Association for the Study of Liver (EASL) Congress in Milan, Italy.
- In April 2024, LyGenesis announced that the first patient had been dosed in their Phase IIa clinical trial evaluating their first-in-class allogenic regenerative cell therapy transplanted into patients' lymph nodes as a potential treatment for end-stage liver disease (ESLD).
- In March 2024, Lipocine announced positive topline results from a Phase II clinical study of LPCN 1148. LPCN 1148 is an oral candidate under development for the clinical management of liver cirrhosis.
Request a sample and discover the recent advances in liver cirrhosis drugs @ Liver Cirrhosis Pipeline Report
The liver cirrhosis pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage liver cirrhosis drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the liver cirrhosis clinical trial landscape.
Liver Cirrhosis Overview
Liver cirrhosis is a condition where the liver becomes scarred and permanently damaged, with healthy liver tissue replaced by scar tissue, impairing its normal function. It often results from long-term liver damage caused by conditions like alcohol-related liver disease, nonalcoholic fatty liver disease, chronic hepatitis C, and chronic hepatitis B. Symptoms may not be noticeable until significant liver damage occurs and can include fatigue, severe itching, and swelling in the legs and abdomen. Doctors diagnose cirrhosis through a combination of medical history, physical examination, and tests such as blood work, imaging, and liver biopsy. While there is no definitive cure, addressing the underlying causes can slow disease progression and reduce the risk of liver failure. Complications may include portal hypertension, infections, and liver cancer. Managing cirrhosis involves eating a healthy diet, avoiding alcohol and liver-damaging foods like raw shellfish, and, in severe cases, considering a liver transplant.
The symptoms of liver cirrhosis can vary based on its severity. Early signs include fatigue, poor appetite, weight loss, nausea, abdominal pain, and spider-like red blood vessels on the skin. As the condition worsens, symptoms may include fluid buildup in the legs and abdomen, yellowing of the skin and eyes (jaundice), redness on the palms, breast swelling and impotence in men, easy bruising, abnormal bleeding, confusion or difficulty thinking, pale stools, and gastrointestinal bleeding.
Diagnosing liver cirrhosis involves blood tests, imaging studies, and, sometimes, a liver biopsy. Blood tests can identify elevated liver enzymes, abnormal liver function, or signs of inflammation or infection. Imaging techniques such as ultrasound, CT scans, or MRIs can reveal liver abnormalities, while a biopsy can confirm the diagnosis and evaluate the extent of damage.
Treatment focuses on slowing scar tissue formation, managing symptoms, and preventing complications. Lifestyle changes may include a healthy diet, stopping alcohol consumption, weight loss for obese individuals, regular exercise, and maintaining good hygiene to lower infection risks. Medications may include antivirals for hepatitis, diuretics to reduce fluid retention, beta-blockers to prevent bleeding from enlarged veins, and creams to relieve itching.
Find out more about liver cirrhosis drugs @ Liver Cirrhosis Analysis
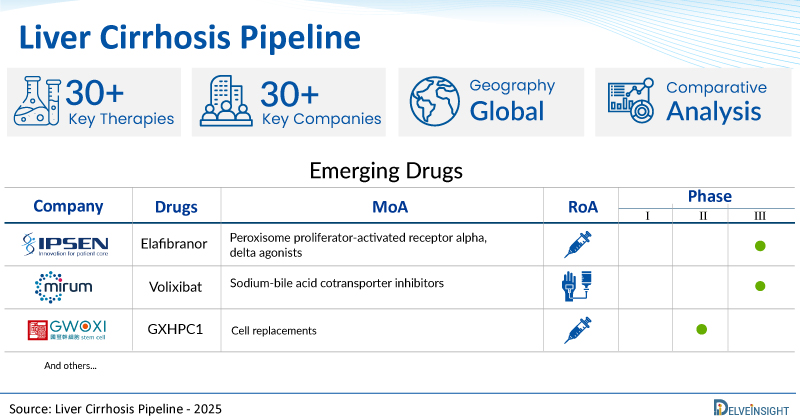
A snapshot of the Pipeline Liver Cirrhosis Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| Elafibranor | Ipsen | III | Peroxisome proliferator-activated receptor alpha, delta agonists | Oral |
| Volixibat | Mirum Pharmaceuticals | III | Sodium-bile acid cotransporter inhibitors | Oral |
| GXHPC1 | Gwo Xi Stem Cell Applied Technology | II | Cell replacements | Intrahepatic |
| RTX-001 | Resolution Therapeutics | II | Macrophage replacements | Intravenous |
| PHIN-214 | PharmaIN | I | Vasopressin 1a receptor agonists | Subcutaneous |
Learn more about the emerging liver cirrhosis therapies @ Liver Cirrhosis Clinical Trials
Liver Cirrhosis Therapeutics Assessment
The liver cirrhosis pipeline report proffers an integral view of the emerging liver cirrhosis therapies segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Liver Cirrhosis Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Intra-articular, Intraocular, Intrathecal, Intravenous, Oral, Parenteral, Subcutaneous, Topical, Transdermal
- Therapeutics Assessment By Molecule Type: Oligonucleotide, Peptide, Small molecule
- Therapeutics Assessment By Mechanism of Action: Sodium-bile acid cotransporter inhibitors, Regulatory T-lymphocyte stimulants, Ammonia scavengers, DNA gyrase inhibitors, DNA topoisomerase inhibitors, DNA-directed RNA polymerase inhibitors, Beta-catenin inhibitors, CREB-binding protein inhibitors, Wnt signalling pathway inhibitors, Bacteria replacements, Microbiome modulators
- Key Liver Cirrhosis Companies: Versantis AG, Mirum Pharmaceuticals, Sagimet Biosciences, TenNor Therapeutics, Prism Pharma, Vedanta Biosciences, Intercept Pharmaceuticals, Boehringer Ingelheim, Ohara Pharmaceutical, Ocelot Bio, Calliditas Therapeutics, Galecto Biotech, Pharmicell, and others.
- Key Liver Cirrhosis Pipeline Therapies: Volixibat, VS-01, TVB-2640, TNP-2092, PRI-724, VE303, Obeticholic Acid (OCA), BI 685509, OP-724, OCE-205, GKT137831, GB1211, Cellgram-LC, and others.
Dive deep into rich insights for new liver cirrhosis treatments, visit @ Liver Cirrhosis Drugs
Table of Contents
| 1. | Liver Cirrhosis Pipeline Report Introduction |
| 2. | Liver Cirrhosis Pipeline Report Executive Summary |
| 3. | Liver Cirrhosis Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Liver Cirrhosis Clinical Trial Therapeutics |
| 6. | Liver Cirrhosis Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Liver Cirrhosis Pipeline: Late-Stage Products (Phase III) |
| 8. | Liver Cirrhosis Pipeline: Mid-Stage Products (Phase II) |
| 9. | Liver Cirrhosis Pipeline: Early-Stage Products (Phase I) |
| 10. | Liver Cirrhosis Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Liver Cirrhosis Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Liver Cirrhosis Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the liver cirrhosis pipeline therapeutics, reach out @ Liver Cirrhosis Therapeutics
Related Reports
Liver Cirrhosis Epidemiology Forecast
Liver Cirrhosis Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted liver cirrhosis epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Liver Cirrhosis Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key liver cirrhosis companies, including Madrigal Pharmaceuticals, Inc., Galectin Therapeutics Inc., Grifols Therapeutics LLC, CymaBay Therapeutics, Akero Therapeutics, Inc., NGM Biopharmaceuticals, Inc., Gilead Sciences, Novo Nordisk A/S, Cellaion, Promethera Therapeutics, Lipocine Inc., Bristol Myers Squibb, Prism Pharma, Ohara Pharmaceutical, Shionogi, Rohto, among others.
Advanced Hepatocellular Carcinoma with CPB Liver Cirrhosis Pipeline
Advanced Hepatocellular Carcinoma with CPB Liver Cirrhosis Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key advanced hepatocellular carcinoma with CPB liver cirrhosis companies, including Can-Fite Biopharma, among others.
Advanced Hepatocellular Carcinoma with CPB Liver Cirrhosis Market
Advanced Hepatocellular Carcinoma with CPB Liver Cirrhosis Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key advanced hepatocellular carcinoma with CPB liver cirrhosis companies, including Can-Fite Biopharma, among others.
Hearing Loss Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key hearing loss companies, including Acousia Therapeutics, Decibel Therapeutics, Otonomy Inc., Sensorion, Autifony Therapeutics, Auris Medical, Sound Pharmaceuticals, Anida Pharma Inc., Gateway Biotechnology, Myrtelle Inc., Lineage Cell Therapeutics, Inc., Altamira Therapeutics, Hoba Therapeutics, Rinri Therapeutics, Autifony Therapeutics, Otologic Pharmaceutics, Audion Therapeutics, Perha Pharmaceuticals, Applied Genetic Technologies Corporation, Akouos, Inc., Oricula Therapeutics, Spiral Therapeutics, Pipeline Therapeutics, Prime Medicine, Boehringer Ingelheim, Autigen, Heyu (Suzhou) Pharmaceutical Technology Co., Ltd, Astellas Pharma, Mogrify Limited, among others.
Nonalcoholic Steatohepatitis Market
Nonalcoholic Steatohepatitis Market Insight, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the market trends, market drivers, market barriers, and key nonalcoholic steatohepatitis companies, including Boehringer Ingelheim, Oramed Pharmaceuticals, ENYO Pharma, Terns Pharmaceuticals, Cirius Therapeutics, among others.
Non-Alcoholic Fatty Liver Disease Market
Non-Alcoholic Fatty Liver Disease Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key non-alcoholic fatty liver disease companies including AstraZeneca, Novartis, Pfizer, Roche, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Contact Us
Shruti Thakur
info@delveinsight.com
+14699457679
www.delveinsight.com
Distribution channels: Banking, Finance & Investment Industry, Healthcare & Pharmaceuticals Industry, Media, Advertising & PR, Science ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release

